39 actavis label
Actavis Logo - LogoDix Find the perfect Actavis logo fast in LogoDix! Search. Actavis Logo. We have found 35 Actavis logos. Do you have a better Actavis logo file and want to share it? We are working on an upload feature to allow everyone to upload logos! 14,660 logos of 489 brands, shapes and colors. Logos by Letter. Logo Categories. Flagyl Online 250 mg or 500 mg Metronidazole Metronidazole is an antibiotic that is used to treat bacterial infections of the vagina, stomach, liver, skin, joints, brain and spinal cord, lungs, heart, or bloodstream. We want to help you find those areas much faster by offering you accessibility to our credibled evaluation web page, with ideal pharmacies ready and standing by.
Hydroxocobalamin: Package Insert - Drugs.com Package insert / product label Dosage form: injection, solution Drug class: Vitamins. Medically reviewed by Drugs.com. Last updated on Nov 21, 2022. On This Page. Description; ... Actavis Pharma, Inc. Parsippany, NJ 07054 USA. PIN229-WAT/2. PRINCIPAL DISPLAY PANEL. NDC 0591-2888-30 30 mL Sterile Multiple Dose Vial Hydroxocobalamin Injection USP
Actavis label
Gabapentin Actavis - Gabapentin uses, dose and side effects Gabapentin Actavis is used to treat: Epilepsy: ... Do not use this medicine after the expiry date which is stated on the carton / label after EXP. The expiration date is the last day of the specified month. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. Actavis: Still the Trusted Name in U.S. Generics - Pharmacy Times Actavis generic products in the U.S. will continue to carry the Actavis name and label. On March 17, 2015, Actavis combined with another industry giant, Allergan, creating one of the world's fastest growing, most dynamic pharmaceutical companies in global health care. Actavis Product Definition | Law Insider Actavis Product. definition. Actavis Product means an extended release oral capsule product containing the Compound as its sole active ingredient, which is the subject of the Actavis ANDA, including all formulations and strengths thereof, described therein now or hereafter. Actavis Product all Products that are being researched, tested ...
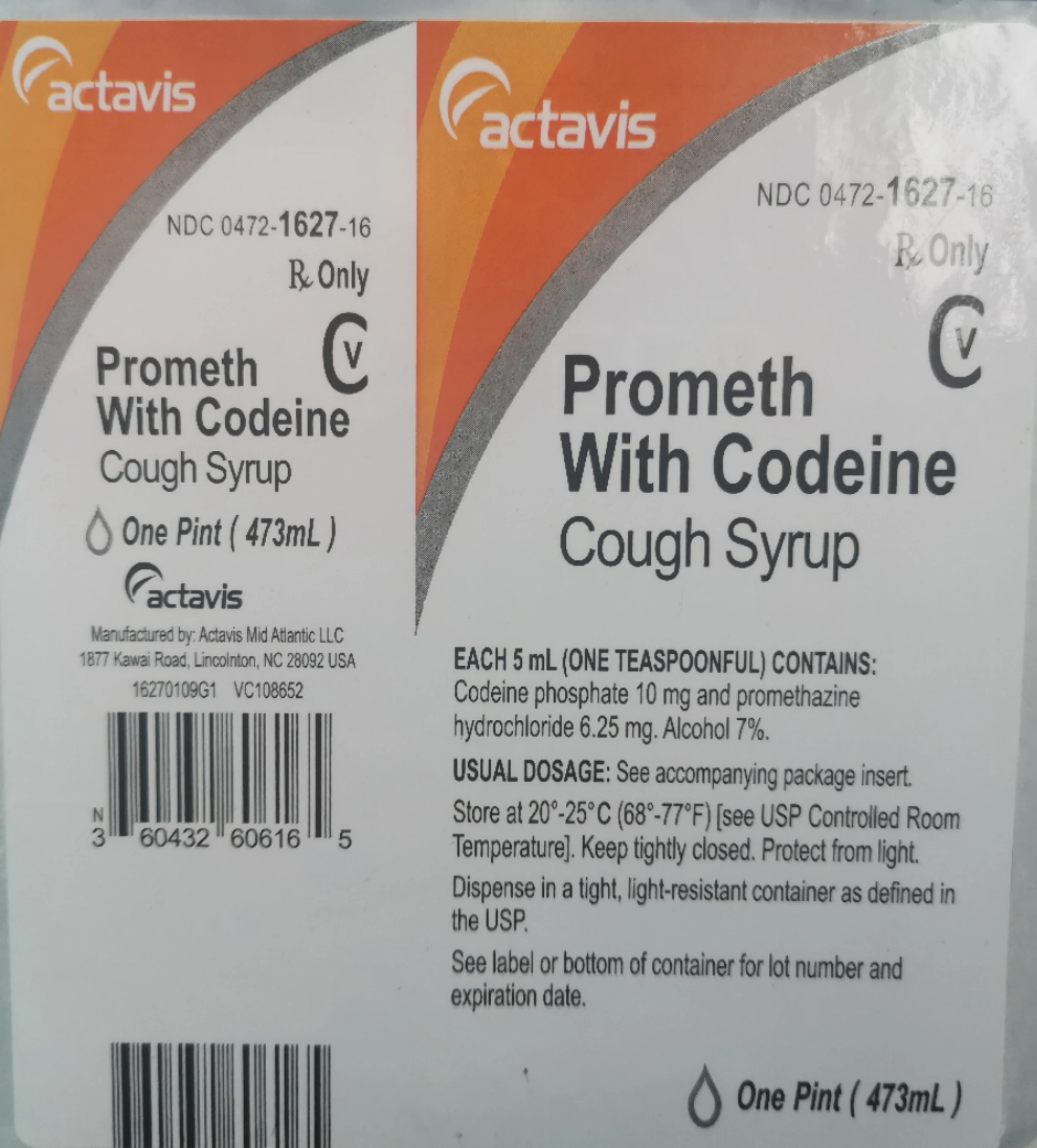
Actavis label. DailyMed - TESTOSTERONE CYPIONATE injection Label: TESTOSTERONE CYPIONATE injection NDC Code (s): 0591-4128-79 Packager: Actavis Pharma, Inc. Category: HUMAN PRESCRIPTION DRUG LABEL DEA Schedule: CIII Marketing Status: Abbreviated New Drug Application Drug Label Information Updated October 18, 2018 If you are a consumer or patient please visit this version. Download DRUG LABEL INFO: PDF XML HZNP Medicines LLC v. Actavis Labs. UT, Inc. | Resources | Robins ... Actavis' label contained a warning to "wait until area is completely dry before covering with clothing or applying sunscreen, insect repellent, cosmetics, topical medications or other substances." The district court found that Actavis' label merely permitted, without encouraging, the application of a second topical medication. DailyMed - PREDNISONE tablet Packager: Actavis Pharma, Inc. Category: HUMAN PRESCRIPTION DRUG LABEL DEA Schedule: None Marketing Status: Abbreviated New Drug Application Drug Label Information Updated October 1, 2015 If you are a consumer or patient please visit this version. Download DRUG LABEL INFO: PDF XML Official Label (Printer Friendly) View All Sections DESCRIPTION Actavis | Uses, Dosage, Side Effects, FAQ - MedicinesFAQ Actavis Actavis Tablet Drugs for Osteoarthritis, Drugs used for Rheumatoid Arthritis, Non-steroidal Anti-inflammatory Drugs (NSAIDs) Actavis is a NSAID that inhibits both isoforms of COX enzyme, a key enzyme involved in the inflammatory cascade.
Logo (Actavis) 2848 Logo (Actavis) 2848 Pill - Drugs.com Pill with imprint Logo (Actavis) 2848 Logo (Actavis) 2848 is Gray / Green, Capsule-shape and has been identified as Fluvoxamine Maleate Extended-Release 100 mg. It is supplied by Actavis Elizabeth LLC. Fluvoxamine is used in the treatment of anxiety and stress; obsessive compulsive disorder; social anxiety disorder and belongs to the drug class ... Who is Actavis Inc & What Medications Do They Sell? - Sunrise House Actavis, Inc. was a global pharmaceutical business that was involved in the development, manufacturing, and distribution of its own brand named pharmaceuticals as well as the manufacture and distribution of generic pharmaceutical products, particularly a number of generic products from a company it had acquired, Watson Pharmaceuticals. Teva Pharmaceuticals USA Issues Voluntary Nationwide Recall of ... Teva Pharmaceuticals USA today confirmed a voluntary recall to the consumer / user level of 29 lots of single and 51 lots of combination valsartan medicines distributed under the Actavis label in... Actavis Phone Number | Call Now & Shortcut to Rep - GetHuman While 800-249-5499 is Actavis's best toll-free number, there are 3 total ways to get in touch with them. The next best way to talk to their customer support team , according to other Actavis customers, is by calling their 800-272-5525 phone number for their Customer Service department. Besides calling, the next favorite option for customers ...
PDF Reference ID: 4152597 - Food and Drug Administration Reference ID: 4152597 3 recommended patients with hemoglobin <10 g/dL. including neutropenia and febrile neutropenia. Monitor closely. 2 2 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do... PDF HIGHLIGHTS OF PRESCRIBING INFORMATION worsening of signs and symptoms ... Recommended dosage is 50 mg to 400 mg administered every two to four weeks as a deep intramuscular injection in the gluteal muscle. Individualize the dose and schedule based on the patient's age,... Teva Pharmaceuticals USA, Inc. Initiates Voluntary Nationwide Recall of ... The lots being recalled are packaged under the Actavis Pharma, Inc. label and are contained in the table below. They were distributed nationwide in the USA as retail bottles of 100 tablets and... Fluvoxamine Maleate (Actavis Pharma, Inc.): FDA Package Insert 3 DOSAGE FORMS AND STRENGTHS. Fluvoxamine maleate extended-release capsules are available as: 100 mg - Each #2 capsule with olive opaque cap and gray opaque body, imprinted with and 2848 on both cap and body in black ink contains 100 mg of fluvoxamine maleate, USP. 150 mg - Each #1 capsule with olive opaque cap and white opaque body ...
Rivastigmine Actavis | European Medicines Agency Rivastigmine Actavis is a medicine containing the active substance rivastigmine. It is available as capsules (1.5, 3, 4.5 and 6 mg). Rivastigmine Actavis is a 'generic medicine'. This means that Rivastigmine Actavis is similar to a 'reference medicine' already authorised in the European Union (EU) called Exelon.
Actavis Product Definition | Law Insider Actavis Product. definition. Actavis Product means an extended release oral capsule product containing the Compound as its sole active ingredient, which is the subject of the Actavis ANDA, including all formulations and strengths thereof, described therein now or hereafter. Actavis Product all Products that are being researched, tested ...
Actavis: Still the Trusted Name in U.S. Generics - Pharmacy Times Actavis generic products in the U.S. will continue to carry the Actavis name and label. On March 17, 2015, Actavis combined with another industry giant, Allergan, creating one of the world's fastest growing, most dynamic pharmaceutical companies in global health care.
Gabapentin Actavis - Gabapentin uses, dose and side effects Gabapentin Actavis is used to treat: Epilepsy: ... Do not use this medicine after the expiry date which is stated on the carton / label after EXP. The expiration date is the last day of the specified month. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required.





![Jual Actavis Apolar Obat [0,5 mg /g Cream] di Seller Hakim ...](https://www.static-src.com/product-review/full//117/777188791.jpg)
























Post a Comment for "39 actavis label"