45 open label study
What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials are used to compare different treatments or gather further information on the long-term effects of new drugs and treatments. In some instances, patients are eligible to... Efficacy and Safety of Evolocumab in Reducing Lipids and Cardiovascular ... On completing a trial of evolocumab (parent trial), patients could enroll into one of two longer-term extension trials, designated Open-Label Study of Long-Term Evaluation against LDL Cholesterol...
Understanding Clinical Trial Terminology: What is an Open Label ... Jun 24, 2019 · Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. In some instances, patients who complete one clinical trial may be eligible to continue in an open-label extension study where all participants are eligible to receive active treatment for an ...

Open label study
PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology For example, in an open-label extension study of etanercept for ankylosing spondylitis, "efficacy analyses are reported without statistical inference between treatment groups"3. Although the CONSORT guideline has provided a frame-work for the design, analysis, and reporting of RCT4, no consensus statement has been developed for open-label End of Trial and Open-Label Extension (OLE) Frequently Asked Questions ... Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given to all participants at the same time and to follow them over time. An Open Label, Randomized, Multicenter Study of Elafibranor in ... - PubMed An Open Label, Randomized, Multicenter Study of Elafibranor in Children with Nonalcoholic Steatohepatitis J Pediatr Gastroenterol Nutr. 2023 Apr 21. doi: 10.1097 ... Methods: Children with NASH were randomized to open-label elafibranor 80mg or 120mg daily for 12 weeks. The intent-to-treat analysis included all participants who received at least ...
Open label study. Reducing bias in open-label trials where blinded outcome assessment is ... Nov 21, 2014 · Many trial designs do not permit blinding, and are therefore designed as open-label, with patients, clinicians, and other study investigators aware of treatment allocation. Research has suggested that these trials should use blinded outcome assessment to avoid bias in estimated treatment effects [6–10]. Blinded outcome assessment is often ... Open-label extension studies: do they provide meaningful ... - PubMed However, this can only occur if the open-label extension study is designed, executed, analysed and reported competently. Most of the value accrued in open-label extension studies is gained from a refinement in the perception of the expected incidence of adverse effects that have most likely already been identified as part of the preclinical and ... What is an open label trial? | The BMJ May 23, 2014 · An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities. What is an Open Label Study in Clinical Research? | Power Open-label study advantages for participants. For participants enrolled in an open-label study, or open-label trial, there is a potential advantage in knowing exactly what treatment you are receiving. To begin, it removes one of the most common uncertainties people express regarding participating in clinical research studies, which is being ...
Savolitinib Plus Osimertinib Versus Platinum-based Doublet Chemotherapy ... Mar 2, 2022 · This is a multicentre, Phase III, randomised, open-label study to investigate the efficacy and safety of savolitinib administered orally in combination with osimertinib versus platinum-based doublet chemotherapy in participants with EGFR mutated, MET-overexpressed and/or amplified, locally advanced or metastatic NSCLC who have progressed on first- or second-line treatment with osimertinib as ... Open Label Study to Evaluate Efficacy and Long Term Safety of LUM001 ... This is an open label study in children with Progressive Familial Intrahepatic Cholestasis (PFIC) designed to evaluate the safety and efficacy of LUM001, also known as Maralixibat (MRX). Efficacy will be assessed by evaluating the effect of LUM001 on pruritus and the biochemical markers of pruritus associated with PFIC. Facebook - National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine. Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, [4] or possible relief from symptoms of some disorders when a placebo is given. [5] An open-label trial may still be randomized.
Open-Label Extension Studies | SpringerLink 1. Open-Label Extension Studies. As the name implies, an open-label extension study is an 'appendage' to a randomised controlled clinical trial, usually of an unregistered medicine or intervention. Often the drug is being studied under an investigational new drug (IND) licence or equivalent legislation. National Center for Biotechnology Information National Center for Biotechnology Information Medical Definition of Open-label - MedicineNet Medical Definition of Open-label. Open-label: A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid ... An open-label study published in 2004 suggested, by comparison with a historical control group that received only ribavirin, that the addition of lopinavir-ritonavir (400 mg and 100 mg ...
Open-Label Placebo Improved Outcomes in Opioid Use Disorder Open-Label Placebo Improved Outcomes in Opioid Use Disorder Study showed placebo increased retention, improved sleep even when participants knew they were taking placebo By JACQUELINE MITCHELL | Beth Israel Deaconess April 17, 2023 Research 3 min read Image: Aleksei Bezrukov/iStock/Getty Images Plus
Open-Label Trial - an overview | ScienceDirect Topics Open-label trials of desipramine, tranylcypromine, reboxetine, and bupropion showed improvement with the drug therapy.55 An open-label trial of escitalopram for SAD patients (N = 20) over 8 weeks produced a response rate of 95% (SIGH-SAD < 50% of baseline value) and a remission rate (SIGH-SAD score < 8) of 85%. 56 In an open-label trial of …
Spotlight on Open-Label Extension Studies Jul 1, 2006 · The stated objective of most OLE studies is to obtain long-term safety and tolerability data. Open-label extension (OLE) studies are common, but they do not receive as much attention as traditional Phase I through Phase IV studies. Enrollment into an OLE study typically follows enrollment into a randomized, blinded, well-controlled main study.
What is an open label extension study? • NCK Pharma Open-label trials may be appropriate for comparing two very similar treatments to determine which is most effective. An open-label trial may be unavoidable under some circumstances, such as comparing the effectiveness of a medication to intensive physical therapy sessions. admin
Open-label study | definition of open-label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc.
An Open Label, Randomized, Multicenter Study of Elafibranor in ... - PubMed An Open Label, Randomized, Multicenter Study of Elafibranor in Children with Nonalcoholic Steatohepatitis J Pediatr Gastroenterol Nutr. 2023 Apr 21. doi: 10.1097 ... Methods: Children with NASH were randomized to open-label elafibranor 80mg or 120mg daily for 12 weeks. The intent-to-treat analysis included all participants who received at least ...
End of Trial and Open-Label Extension (OLE) Frequently Asked Questions ... Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given to all participants at the same time and to follow them over time.
PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology For example, in an open-label extension study of etanercept for ankylosing spondylitis, "efficacy analyses are reported without statistical inference between treatment groups"3. Although the CONSORT guideline has provided a frame-work for the design, analysis, and reporting of RCT4, no consensus statement has been developed for open-label













![PDF] Bias was reduced in an open-label trial through the ...](https://d3i71xaburhd42.cloudfront.net/814ad6ccbfa9defca4d2b00c4672f9070cf6b8da/16-Figure1-1.png)



















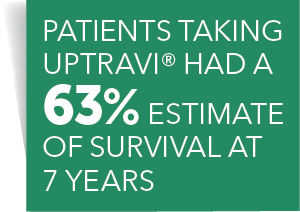
Post a Comment for "45 open label study"